A Six-Month Clinical Study to Evaluate the Effects of Sodium Tripolyphosphate and Tetrapotassium Pyrophosphate Based Calculus Dissolution Oral Rinse in Patients with Zirconium Dioxide and Titanium Dental Implants
DOI:
https://doi.org/10.5530/BEMS.2016.2.11Keywords:
Dental implant, Oral rinse, Anti-calculus, Peri-implantitis, Plaque index, Biopolymers, Clinical trialAbstract
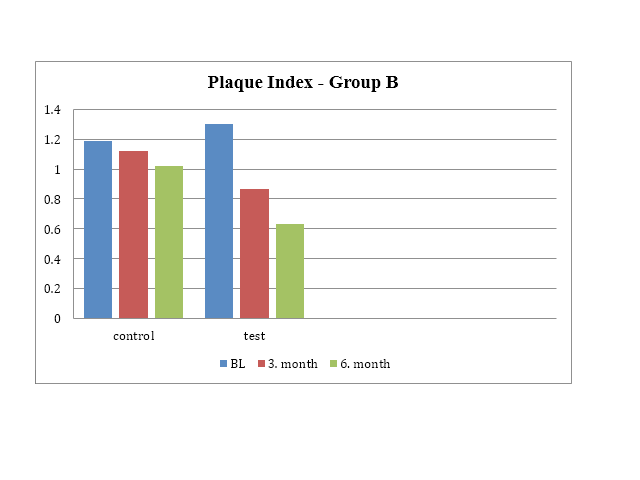
Aim: To clinically evaluate the effects of a novel anti-calculus mouth rinse containing sodium tripolyphosphate and tetrapotassium pyrophosphate (Periogen, USA) on development of gingivitis and plaque around dental implants versus control over a period of six months. Material and methods: This was a randomized, 6 month, parallel groups, double blind, single center clinical trial. Forty subjects with present dental implants (22 zirconium dioxide and 20 titanium) were randomly assigned to one of two subgroups: control group (regular brushing) and test group (regular brushing followed by using the calculus dissolution based oral rinse). All subjects were assessed with gingival index (GI), plaque index (PI) and Volpe-Manhold calculus index (VMI) after 3 and 6 months. Results: Statistical analysis found that test group in both zirconium dioxide and titanium group demonstrated statistically significant lower GI, PI and VMI scores. Conclusion: This study demonstrates that calculus dissolution based Periogen ® mouthrinse provided clinically significant reduction in calculus formation in subjects with zirconium dioxide and titanium dental implants when used twice daily for 6 months as an adjunct to toothbrushing.